Technology summary
Neurological and cognitive disorders such as epilepsy, pain, fear and anxiety, depression, addiction, apnea and tremors can result from aberrant activity of defined subsets of neurons, including an imbalance of excitatory versus inhibitory signaling. However, it is difficult to alter neuron function selectively in the brain for research or clinical applications.
To address this critical need, Dr. Zemelman and his group have developed a suite of genetic reagents to identify and access key neuron subclasses in mammals, including primates, for clinical and research applications. The reagents include activity-dependent reporters to mark dysfunctional neurons1 and viral vectors with engineered regulatory elements to target defined neuron populations 2. The Zemelman lab is utilizing these tools to illuminate cell and circuit-level mechanisms of memory, auditory function, and visual processing.
To Dr. Zemelman, an optogenetics pioneer,3 these new tools are essential to be able to alter brain function narrowly in a clinical setting. The reagents can also be utilized to identify critical populations of neurons for drug screening and to monitor treatment efficacy real-time. In recent studies, the Zemelman Lab and their collaborators have accessed all excitatory and inhibitory neurons as well as the somatostatin, parvalbumin, cholecystokinin, and neuropeptide-Y-positive subsets of neurons across mammalian models, including primates, to study forebrain neuron structure-function relationships. Several patent applications have been filed, and UT Austin seeks an industry partner to further develop the technology for research and therapeutic applications.
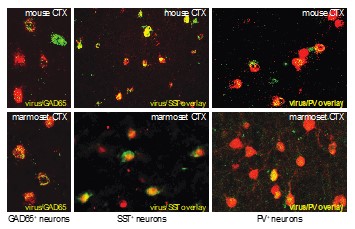
Fig. 1. Cell type specific targeting of GABAergic interneurons in the rodent and primate neocortex. Novel virus-based promoters were used to access all GABAergic neurons as well as somatostatin (SST) and parvalbumin-positive (PV) inhibitory neuron subclasses. In these examples, cell identify was confirmed using in situ hybridization in the top panels and marmoset GAD65, and by immunostaining for marmoset SST and PV.
Key advantages
- Genetic tools target previously inaccessible neuronal subclasses
- Short, highly specific promoters achieve high levels of transgene expression
- Validated in primates
- Applicable to selective gene therapies for neurological and neuropsychiatric disorders
- Applicable for early-stage drug candidate validation and efficacy screening
1. Zemelman B.V. U.S. Patent Application US20180363003A1
2. Mehta et al., Functional access to neuron subclasses in rodent and primate forebrain. Cell Rep. 26, 2818-2832.e8 (2019).
3. Missenbock, G. and Zemelman B.V. U.S. Patents 7883846 and 7144733

