Background
A separator in a rechargeable battery serves as a physical barrier between the two electrodes, allowing ionic transport with a liquid electrolyte while blocking electrical transport, preventing a short circuit. The separator is the most expensive component by weight of a commercial lithium-ion battery. Additionally, it is the least researched component of modern rechargeable batteries. If the price per weight of the separator is reduced, the energy and power density of a battery can be increased.
Technology
Researchers at The University of Texas at Austin have developed an electrode coating that can serve as a separator for batteries. The coating is a self-assembly of nanoparticles, making the components easy to manipulate for processing. The raw materials for the coating are cheap and easy to synthesis, making them an ideal candidate for easy implementation in existing battery manufacturing infrastructure. Further development and optimization of the materials and post-processing of these coatings can be performed to tailor performance and properties for different types of batteries in different types of applications.
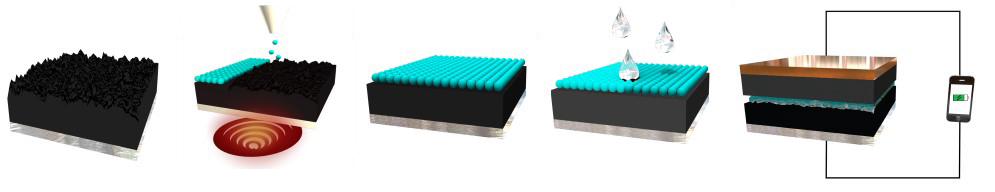
Fig. 1: Coating and assembly process
Figure 1 demonstrates a simple coating and assembly process for the separator. There is sufficient data collected to demonstrate cell performance with a traditional LiCoO2 cathode and a graphite anode with an organic-liquid electrolyte. The resistance of the cell with a self-assembled separator demonstrates a smaller cell resistance than that of a cell with a traditional polymer composite separator owing to the superior wettability of the nanoparticles by the liquid electrolyte.

